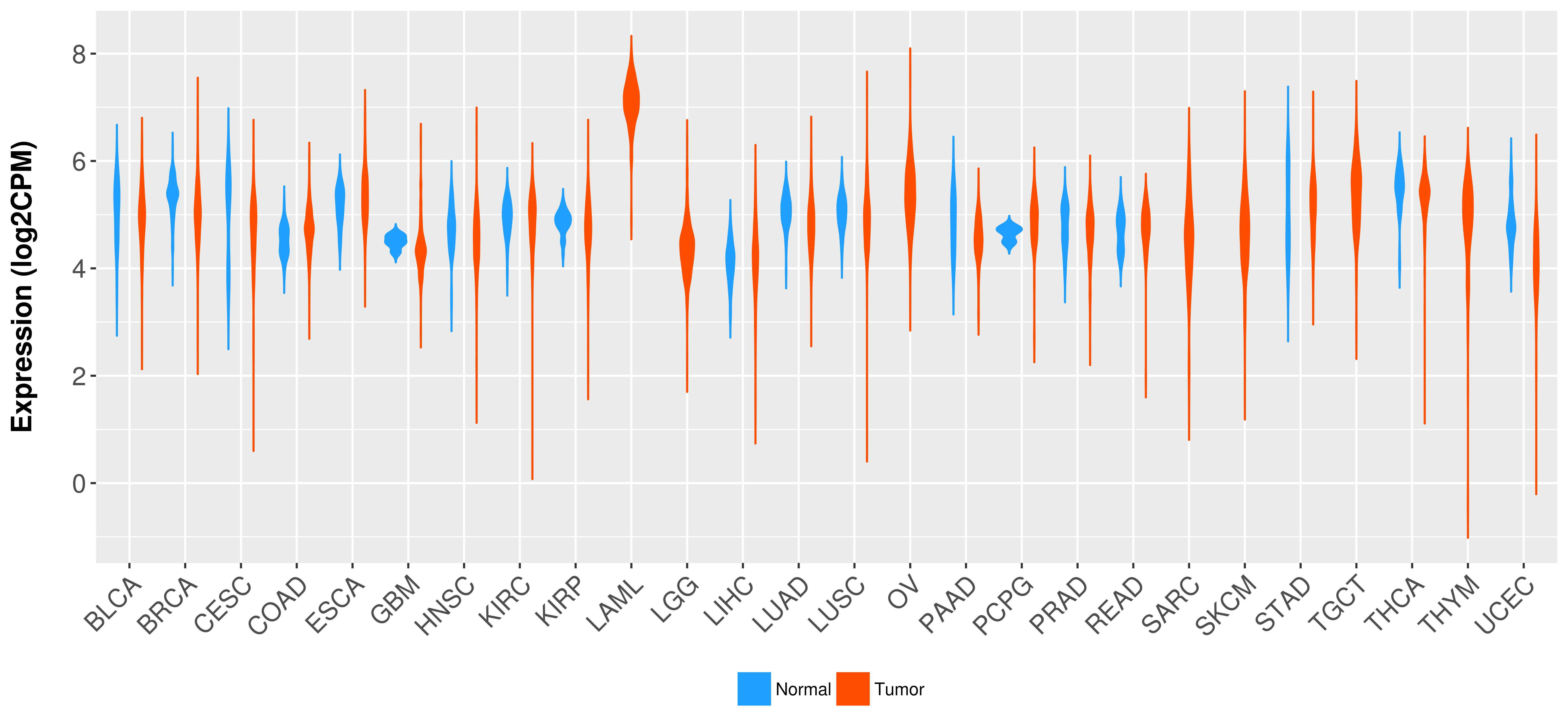
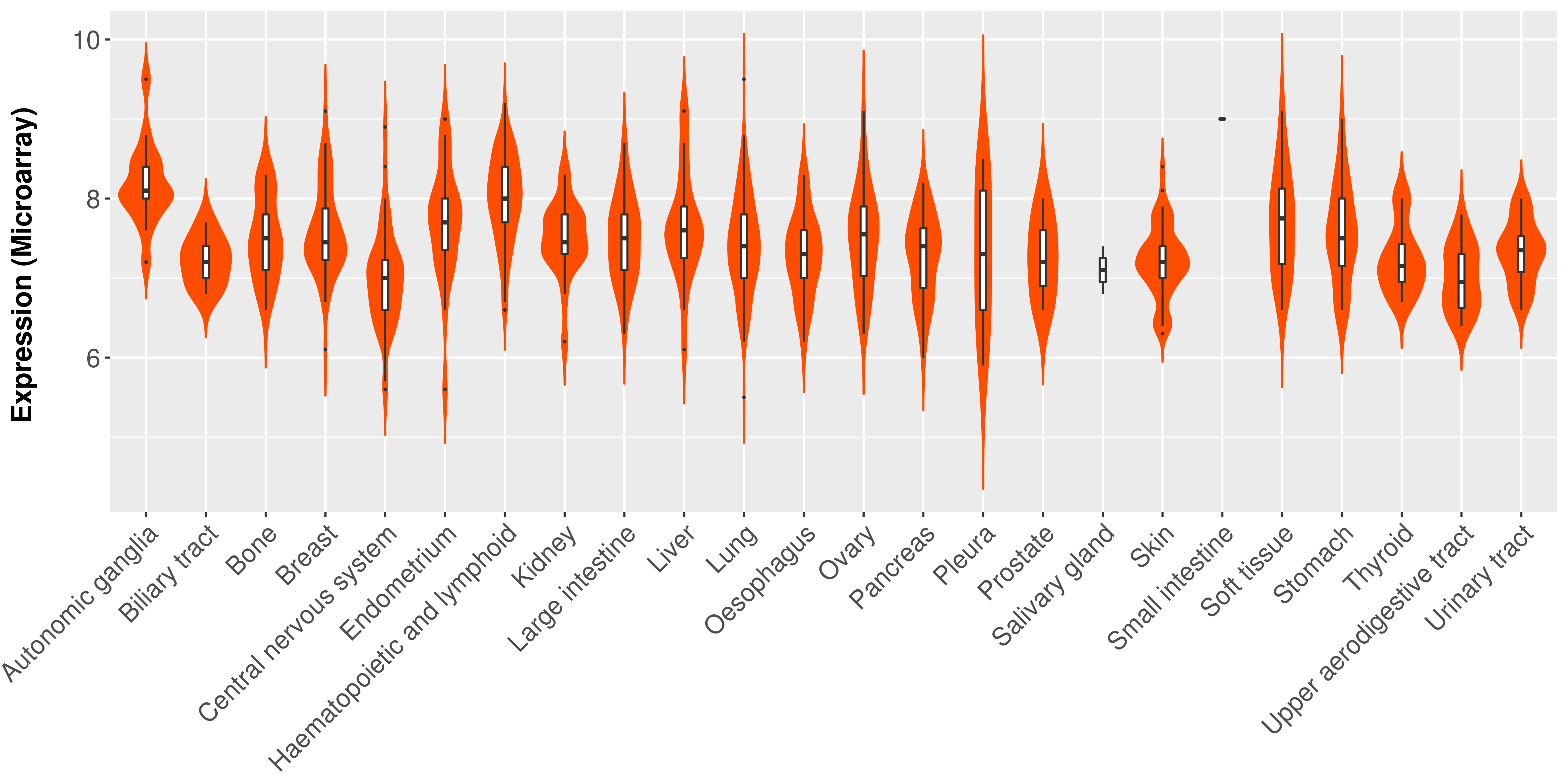
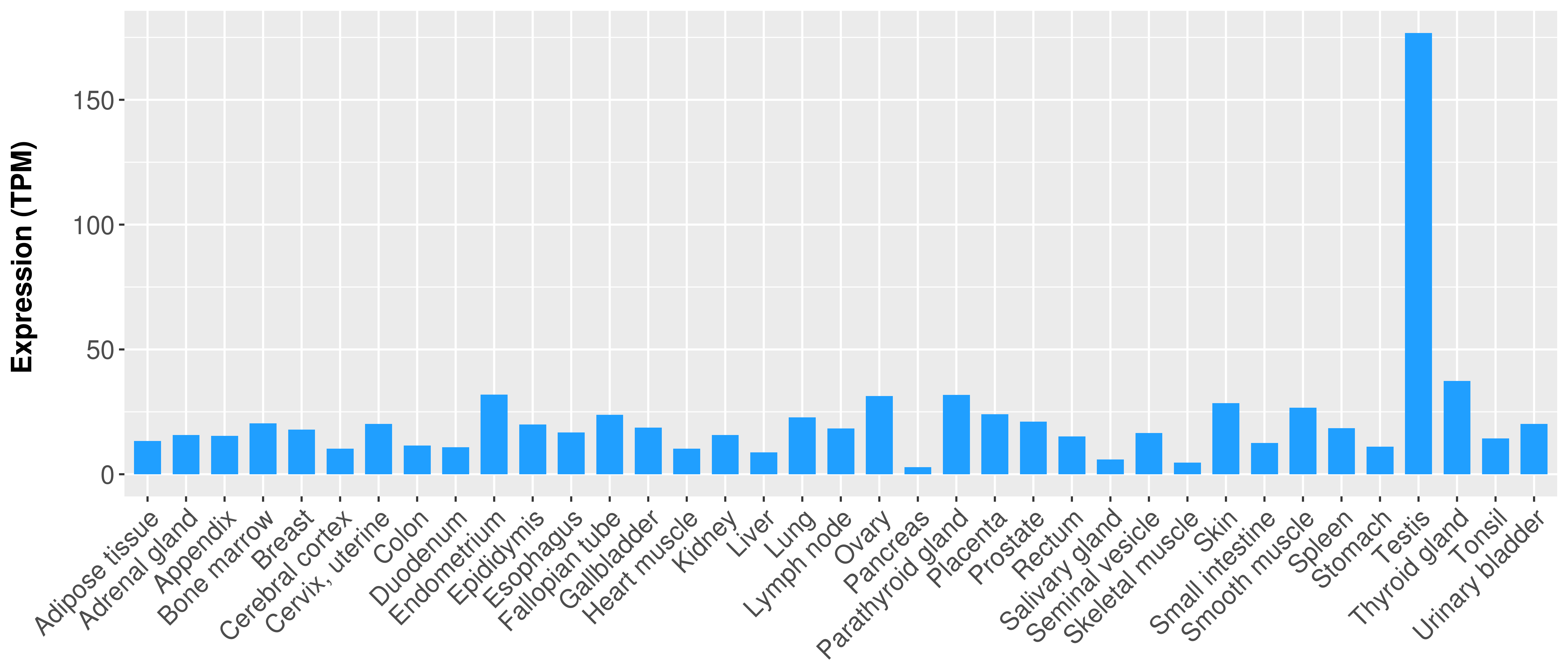
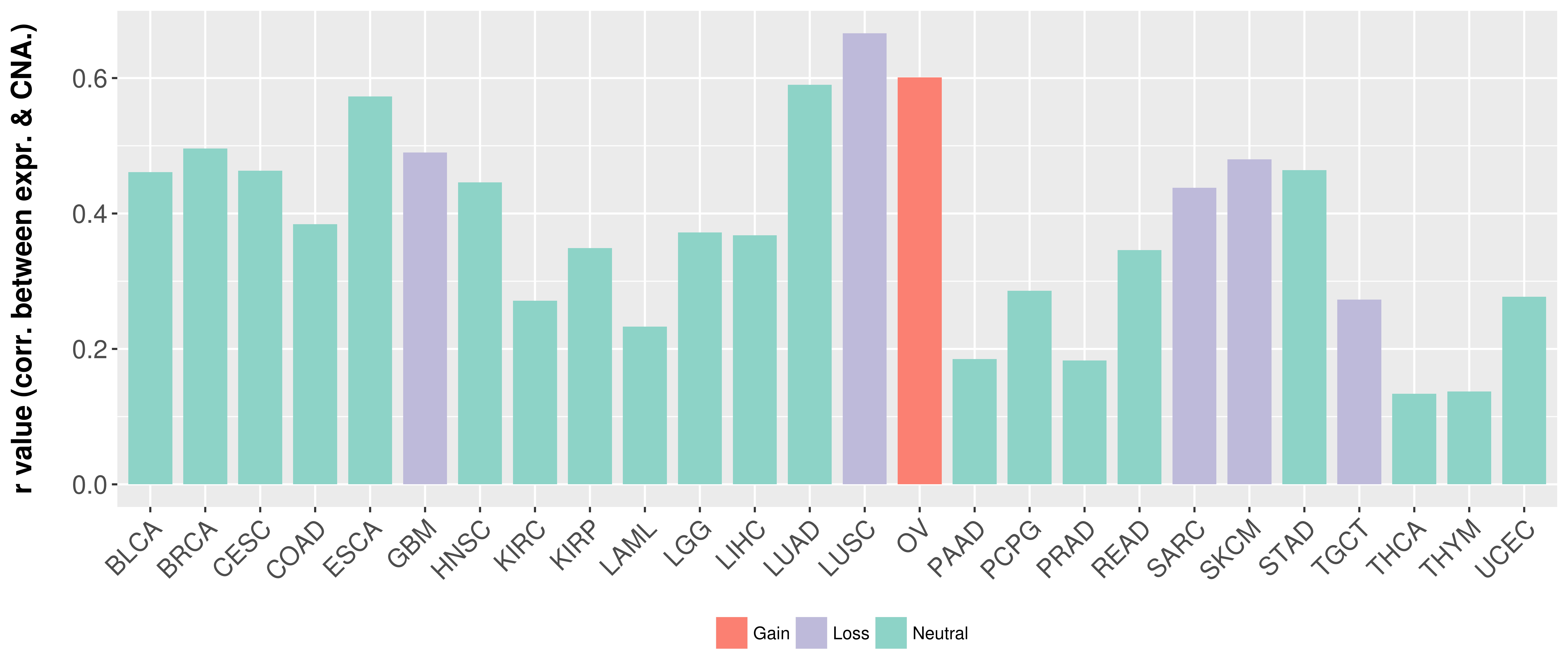
| 27282883 | Mutation | Acute Lymphoblastic Leukemia | Of the KMT2A fusion partner genes, MLLT3 was present in five patients, all with acute lymphoblastic leukemia, MLLT1 in two patients, and MLLT10, MLLT4, MLLT11, and AFF1 in one patient each
|
| 26477762 | Mutation | mixed lineage Leukemia | The MLL-AF10 positive patients were mostly young men, the majority FAB classification was M5 or M4, often onset with fever, low white blood cells and low level of fusion gene, usually associated with WT1 mutation.
|
| 25740345 | Mutation | Leukemia | Chromosomal rearrangements of the MLL gene are associated with high-risk infant, pediatric, adult, and therapy-induced acute leukemias. So far, about 80 different direct MLL fusions and about 120 reciprocal MLL fusions have been characterized at the molecular level.
|
| 25725124 | Mutation | Acute Myeloid Leukemia | Here we investigated an 11-month-old male presenting with hyperleukocytosis being diagnosed with AML subtype FAB-M5b. In banding cytogenetics a der(19)t(19;)(q13.3;) and del(Y)(q11.23) were found as sole aberrations. Molecular cytogenetics revealed that the MLL gene was disrupted and even partially lost due to a t(10;19;11)(p12.31;q13.31;q23.3), an MLL/MLLT10 fusion appeared, and the der(Y) was an asymmetric inverted duplication with breakpoints in Yp11.2 and Yq11.23.
|
| 25464900 | Mutation | Acute Lymphoblastic Leukemia | In the present study, a 16-year-old male was diagnosed with T-precursor cell ALL and a normal karyotype after standard GTG-banding, was studied retrospectively (>10 years after diagnosis) in frame of a research project by molecular approaches. In addition to molecular cytogenetics, multiplex ligation-dependent probe amplification (MLPA) and high resolution array-comparative genomic hybridization (aCGH) were also applied. Thus, the following yet unrecognized balanced chromosomal aberrations were detected: der(3)t(3;5)(p23;q31.1), der(5)t(3;5)(p23;q35.3), der(5)t(5;10)(q31.1;p12.3) and der(10)t(5;10)(q35.3;p12.3). The oncogene MLLT10 was involved in this rearrangement as was the IL3 gene; in addition, trisomy 4 was present. All of these clonal aberrations were found in 40% of the cells.
|
| 25027513 | Mutation | Leukemia | Elevated expression of HOXA genes is critical for leukemia maintenance and progression; however, the precise mechanism by which CALM-AF10 alters HOXA gene expression is unclear. We previously determined that CALM contains a CRM1-dependent nuclear export signal (NES), which is both necessary and sufficient for CALM-AF10-mediated leukemogenesis. Here, we find that interaction of CALM-AF10 with the nuclear export receptor CRM1 is necessary for activating HOXA gene expression. We show that CRM1 localizes to HOXA loci where it recruits CALM-AF10, leading to transcriptional and epigenetic activation of HOXA genes.
|
| 24635731 | Mutation | Lymphoblastic Lymphoma | Fluorescence in situ hybridization screening of paraffin-embedded formalin-fixed tissue indeed revealed the presence of an MLL rearrangement. The genomic fusion partner was identified as AF10 by DNA sequencing of the MLL breakpoint region. The MLL-AF10 fusion gene was further detected in cytologically normal pretreated bone marrow.
|
| 24367274 | Mutation | Acute Lymphoblastic Leukemia | Finally, we detected gene fusions, of which several can explain the over-expression of key driver genes such as TLX1, PLAG1, LMO1, or NKX2-1; and others result in novel fusion transcripts encoding activated kinases (SSBP2-FER and TPM3-JAK2) or involving MLLT10.
|
| 23831922 | Mutation | T Acute Lymphoblastic Leukemia | CALM-AF10 fusion within early T-cell precursor acute lymphoblastic leukemia (21%) did, however, identify a group with a poor prognosis with regards to event-free survival (P=0.04).
|
| 23673860 | Mutation | T Acute Lymphoblastic Leukemia | The MLLT10 gene, located at 10p13, is a known partner of MLL and PICALM in specific leukemic fusions generated from recurrent 11q23 and 11q14 chromosome translocations. Deep sequencing recently identified NAP1L1/12q21 as another MLLT10 partner in T-cell acute lymphoblastic leukemia (T-ALL).
|
| 23628958 | Mutation | Leukemia | All patients were classified according to their gender (852 females and 745 males), age at diagnosis (558 infant, 416 pediatric and 616 adult leukemia patients) and other clinical criteria. Combined data of our study and recently published data revealed a total of 121 different MLL rearrangements, of which 79 TPGs are now characterized at the molecular level. However, only seven rearrangements seem to be predominantly associated with illegitimate recombinations of the MLL gene (≈ 90%): AFF1/AF4, MLLT3/AF9, MLLT1/ENL, MLLT10/AF10, ELL, partial tandem duplications (MLL PTDs) and MLLT4/AF6, respectively.
|
| 22871473 | Mutation | Acute Myeloid Leukemia | In this study, we assessed the characteristics and outcome of 18 PICALM-MLLT10 AML patients. As compared with non PICALM-MLLT10 patients (n=72), PICALM-MLLT10 AML were characterized by more frequent extramedullary diseases, CD7 expression and higher platelet counts.
|
| 22064352 | Mutation | Leukemia | We analyzed the fusion transcripts in 20 new cases of CALM/AF10-positive leukemias, and compared the gene expression profile of 10 of these to 125 patients with other types of leukemia and 10 normal bone marrow samples.
|
| 21880637 | Mutation | T Acute Lymphoblastic Leukemia | We analyzed the incidence and prognostic value of PHF6 mutations in 96 Chinese patients with T-cell acute lymphoblastic leukemia. NOTCH1 mutations, FBXW7 mutations, WT1 mutations, JAK1 mutations, SIL-TAL1 fusions, SET-NUP214 fusions and CALM-AF10 fusions were present in 44/96 (45.8%), 9/96 (9.4%), 4/96 (4.1%), 3/49 (6.1%), 9/48 (18.8%), 3/48 (6.3%) and 0/48 (0%) of patients, respectively.
|
| 20875875 | Mutation | acute myeloblastic Leukemia | A 27-year-old woman was diagnosed with AML with the PICALM-MLLT10 fusion gene.
|
| 20494936 | Mutation | Acute Lymphoblastic Leukemia | We demonstrated that high expression of miR-196b is not unique to MLL-rearranged acute lymphoblastic leukemia but also occurs in patients with T-cell acute lymphoblastic leukemia patients carrying CALM-AF10, SET-NUP214 and inversion of chromosome 7.
|
| 20445327 | Mutation | Acute Lymphoblastic Leukmia; Acute Myeloid Leukemia | Because the CALM-AF10 rearrangement is a rare chromosomal abnormality, it is not included in routine molecular tests for acute leukemia. Here, we describe the cases of 2 patients with the CALM-AF10 fusion gene.
|
| 20362230 | Mutation | Leukemia | Here we describe a new MLL fusion partner gene, NEBL, which was identified in a case of acute myeloid leukemia in an infant. The chromosomal breakpoints of the MLL-NEBL and NEBL-MLL fusion genes were cloned by long-distance inverse polymerase chain reaction. The chromosomal breakpoints were located at 10p12, approximately 570 kb telomic of the MLLT10 (AF10) gene. AF10 and NEBL are localized in such close vicinity that they cannot be distinguished cytogenetically by G banding.
|
| 20299091 | Mutation | mixed phenotype acute Leukemia; bilineal and biphenotypic immunophenotype | We report a case of a 6-month-old boy with a mixed phenotype acute leukemia (MPAL), bilineal and biphenotypic immunophenotype (B-lymphoid lineage and combined B-lymphoid and monocytic lineage) with t(10;11)(p12;q23);MLL-MLLT10.
|
| 20065082 | Mutation | T Acute Lymphoblastic Leukemia | The combined hybridization was abnormal in 21/23 patients (91%), and revealed multiple genomic changes in 13 (56%). It found abnormalities known to be associated with T-cell acute lymphoblastic leukemias, i.e. CDKN2A-B/9p21 and GRIK2/6q16 deletions, TCR and TLX3 rearrangements, SIL-TAL1, CALM-AF10, MLL-translocations, del(17)(q12)/NF1 and other cryptic genomic imbalances, i.e. 9q34, 11p, 12p, and 17q11 duplication, del(5)(q35), del(7)(q34), del(9)(q34), del(12)(p13), and del(14)(q11).
|
| 19840468 | Mutation | B Acute Lymphoblastic Leukemia | 4 kind partner genes of MLL-AF4, AF9, AF10 and ENL were detected.
|
| 19443658 | Mutation | Leukemia | In this study, we show that the CALM-AF10 fusion protein can also greatly reduce global H3K79 methylation in both human and murine leukemic cells by disrupting the AF10-mediated association of hDOT1L with chromatin.
|
| 18299449 | Mutation | T Acute Lymphoblastic Leukemia | One subgroup, including MLL-rearranged, CALM-AF10 or inv (7)(p15q34) patients, is characterized by elevated expression of HOXA genes.
|
| 18245528 | Mutation | T lymphoblastic Lymphoma | Fourteen intermediate LBL (Int-LBL) showed complete TCRD VDJ and TCRG VJ rearrangement, with TCRB VDJ rearrangement in the majority. All Int-LBL expressed HOX11/TLX1 or HOXA9 transcripts and a proportion of the latter were associated with CALM-AF10 or NUP214-ABL fusion transcripts.
|
| 17928886 | Mutation | T Acute Lymphoblastic Leukemia | TAL1 or HOX11L2 rearrangements were associated with trends to good and poor outcomes, respectively. Also cases with high vs low TAL1 expression levels demonstrated a trend toward good outcome. Most cases with lower TAL1 levels were HOX11L2 or CALM-AF10 positive.
|
| 17927867 | Mutation | Acute Myeloid Leukemia | Fusion genes, including AML1/ETO, PML/RARalpha, CBFbeta/MYH11, MLL gene rearrangements (that is, MLL/AF6, MLL/AF9, MLL/AF10, and MLL/MLL), DEK/CAN, TEL/PDGFR, and AML1/MDS1 (EVI-1), were detected in 28 (46.7%) patients by multiplex RT-PCR.
|
| 17459201 | Mutation | Leukemia | Fourteen fusion genes, including PML/RARalpha, PLZF/RARalpha, BCR/ABL, MLL/AF1, MLL/AF6, MLL/AF10, AML/Eto, CBFbeta/MYH11, TLS/ERG, TEL/AML1, MOZ/CBP, MLL/hCDCrel, LAF4/MLLT2, and FIP1L1/PDGFRalpha, and activation of all 4 proto-oncogenes were found in the 31 samples.
|
| 17039236 | Mutation | Acute Leukemia | Among nine cases of acute leukemia with translocation breakpoints at 10p13 and 11q14-21, a CALM/AF10 rearrangement was found in seven and was confirmed by reverse transcriptase polymerase chain reaction (RT-PCR) in all.
|
| 16956820 | Mutation | T Acute Lymphoblastic Leukemia | Our data on CALM-AF10 rearranged T-ALL, albeit based on only three patients, suggest that this type of leukemia is associated with a poor outcome.
|
| 16518848 | Mutation | Leukemia | We also identified overexpression of HOXA9, a gene essential to myeloid differentiation that is expressed in PICALM-MLLT10 and MLL-rearranged acute leukemias.
|
| 16511515 | Mutation | Leukemia | The precise localization of genomic breakpoints within the MLL gene and the involved translocation partner genes (TPGs) was determined and several new TPGs were identified. The combined data of our study and published data revealed a total of 87 different MLL rearrangements of which 51 TPGs are now characterized at the molecular level. Interestingly, the four most frequently found TPGs (AF4, AF9, ENL and AF10) encode nuclear proteins that are part of a protein network involved in histone H3K79 methylation.
|
| 16215946 | Mutation | Acute Lymphoblastic Leukemia | Of the 62 patients, 23(37.1%) were found to carry 13 different fusion genes. The patients with immunophenotype of Pre-B-ALL were found to carry: TEL/AML1(3 cases); E2A/PBX1, E2A/HLF, TLS/ERG, MLL/AF4, MLL/AF9, MLL/AF10, MLL/AFX-MLL/AF6-MLL/ELL, MLL/AF6-MLL/ELL, dupMLL (one case for each); and HOX11 (6 cases).
|
| 16107895 | Mutation | Leukemia | The t(10;11)(p13;q14-21) is found in T-ALL and acute myeloid leukemia and fuses CALM (Clathrin-Assembly protein-like Lymphoid-Myeloid leukemia gene) to AF10. In order to gain insight into the transcriptional consequences of this fusion, microarray-based comparison of CALM-AF10+ vs CALM-AF10- T-ALL was performed. This analysis showed upregulation of HOXA5, HOXA9, HOXA10 and BMI1 in the CALM-AF10+ cases.
|
| 15968309 | Mutation | Leukemia | Ten fusion genes were detected in 115 cases of leukemia, including AML1/ETO, PML/RAR alpha, PLZF/RAR alpha, dupMLL, MLL/AF6, MLL/AF10, CBFbeta/MYH11, BCR/ABL, Hox11, and EVI1 BCR/ABL was positive in all the 52 cases of chronic myeloid leukemia; PML/RAR alpha was found in 21 of 25 acute promyelocytic leukemia (APL), and PLZF/RAR alpha was detected in one case of APL.
|
| 15851025 | Mutation | Leukemia | We demonstrate that direct fusion of hDOT1L to MLL results in leukemic transformation in an hDOT1L methyltransferase activity-dependent manner. Transformation by MLL-hDOT1L and MLL-AF10 results in upregulation of a number of leukemia-relevant genes, such as Hoxa9, concomitant with hypermethylation of H3-K79.
|
| 15843827 | Mutation | Leukemia | Using cDNA microarrays, we determined the gene expression profiles of 40 cell lines as well as of primary leukemias harboring 11q23/MLL rearrangements, t(1;19)[TCF3/PBX1], t(12;21)[ETV6/RUNX1], t(8;21)[RUNX1/CBFA2T1], t(8;14)[IGH@/MYC], t(8;14)[TRA@/MYC], t(9;22)[BCR/ABL1], t(10;11)[PICALM/MLLT10], t(15;17)[PML/RARA], or inv(16)[CBFB/MYH11].
|
| 15637138 | Mutation | T Acute Lymphoblastic Leukemia | Representative patients with T-ALL (91 patients) were classified into surface T-cell receptor (TCR)-expressing T-ALL patients (TCRalphabeta+ or TCRgammadelta+), pre-alphabeta T-ALL patients (cTCRbeta+, TCR-), and immature (IM) cTCRbeta-, TCR- T-ALL patients; 81 patients underwent genotyping for SIL-TAL1, CALM-AF10, HOX11, and HOX11L2.
|
| 15631658 | Mutation | Leukemia | In order to determine the involvement of CALM-AF10 fusion transcripted in primary leukemias with t(10;11) and its chemotherapy sensitivity in vitro, the AF10-CALM fusion transcripts were detected by reverse transcription-polymerase chain reaction (RT-PCR), and the chemotherapy sensitivity testing in vitro was undergone by MTT assay in five t(10;11) leukemia samples from patients with ALL, AML and lymphoblastic lymphoma. The results showed that five different-sized AF10-CALM product and four different-sized CALM-AF10 products were detected.
|
| 15381373 | Mutation | Acute Lymphoblastic Leukemia | We report the case of an 11-month-old patient with a clinical diagnosis of infantile acute lymphoblastic leukemia and an MLL (11q23) rearrangement in 69% of nuclei, revealed with interphase fluorescence in situ hybridization (FISH). These both resulted in a large derivative chromosome 10 and transcription of an MLL/MLLT10 fusion product.
|
| 15355694 | Mutation | Acute Lymphoblastic Leukemia | Of the 50 childhood ALL patients, 18 (36.0%) carried 11 types of fusion genes including E2A/PBX1, TEL/AML1, TLS/ERG, MLL/AF4, MLL/AF9, MLL/AF10, MLL/AFX, MLL/AF6, MLL/ELL, TAL1D, and HOX11, revealed by multiplex RT-PCR, and in 48 cases, 24 (57.1%) had chromosome abnormalities.
|
| 15262427 | Mutation | acute monoblastic Leukemia | We present two new pediatric cases (French-American-British type M5) with MLL-MLLT10 fusion, which we studied with fluorescence in situ hybridization.
|
| 14676124 | Mutation | Pancreatic Neuroendocrine Neoplasm | Sixty-six transcripts were overexpressed > or =3-fold in PENs compared with normal islet cells, including putative oncogenes (MLLT10/AF10), growth factors [insulin-like growth factor-binding protein 3 (IGFBP3)], cell adhesion and migration molecules (fibronectin), and endothelial elements (MUC18/MelCAM and CD31).
|
| 12859878 | Mutation | Leukemia | Of the 191 leukemic samples, 86 (45.0%) carried 14 types of fusion genes including SIL/TAL1, MLL/AF1q, E2A/PBX1, MLL/AF6, AML1/ETO, MLL/AF9, TEL/ABL, BCR/ABL, MLL/AF10, dupMLL, MLL/ENL, TEL/AML1, PML/RARalpha and CBFbeta/MYH11.
|
| 12676784 | Mutation | T Acute Lymphoblastic Leukemia | CALM-AF10 is therefore the most common fusion protein in T-ALL.
|
| 12461747 | Mutation | Acute Myeloid Leukemia | We used panhandle PCR to determine that this rearrangement juxtaposed the MLL (Mixed-Lineage Leukemia) gene to the CALM (Clathrin Assembly Lymphoid Myeloid leukemia) gene at 11q14-q21. The CALM protein participates in recruitment of clathrin to internal membrane surfaces, thereby regulating vesicle formation in both endocytosis and intracellular protein transport. Intriguingly, CALM has been identified in other cases of AML as a translocation partner for the AF10 gene, which has independently been found to be an MLL partner in AML. We identified the MLL-CALM fusion transcript (but not the reciprocal CALM-MLL transcript) in leukemia cell RNA by RT-PCR.
|
| 12127405 | Mutation | acute monocytic Leukemia | Fluorescence in situ hybridization (FISH) analysis in a case of infant acute monocytic leukemia M5 revealed a complex rearrangement between chromosomes 10 and 11, leading to the disruption of the MLL gene. Using two painting probes for chromosomes 10 and 11 and a specific probe for the MLL gene localized on 11q23, we observed a paracentric inversion of the 11q13-q23 fragment translocated to 10p12. Molecular analysis showed that AF10 localized on 10p12 was the fusion partner gene of MLL in this rearrangement (10;11).
|
| 11911106 | Mutation | Acute Myeloid Leukemia | To determine the incidence of the mixed lineage leukemia (MLL) gene rearrangements in acute myeloid leukemia (AML) without cytogenetically-detected 11q23 abnormalities, we screened 64 cases of AML at diagnosis for MLL rearrangement by FISH. One further case with a cytogenetic abnormality close to 11q23 was studied; it was found to have a t(10;11)(p13;q21), and the breakpoints were shown by FISH to involve the Clathrin Assembly Lymphoid Myeloid (CALM) gene at 11q21 and the AF10 gene at 10p13.
|
| 11896537 | Mutation | Acute Myeloid Leukemia | In this study, we describe a detailed molecular cytogenetic analysis of MLL-MLLT10 positive 10;11 rearrangements in two patients. We observed an as yet unreported chromosomal mechanism with at least four breakpoints, leading to MLL-MLLT10 gene fusion in a 24-year-old male.
|
| 11477655 | Mutation | Acute Leukemia | To clarify the clinical features of t(10;11)-leukemias, we investigated 6 samples from acute leukemia patients with t(10;11) and MLL rearrangement and detected MLL-AF10 chimeric transcripts in 5 samples and MLL-ABI1 in one.
|
| 11187895 | Mutation | Leukemia | The MLL-AF10 fusion produced by the t(10;11)(p12;q23) or ins(10;11)(p12;q23q13) occurs in a small percentage of acute leukemias, most commonly acute myelogenous leukemia (AML) of the M5 FAB subtype. We report two cases of AML (M5a and M0) and one case of acute lymphoblastic leukemia containing MLL-AF10 fusion. Each case had varied clinical characteristics, despite expressing similar MLL-AF10 fusion transcripts. Including the three cases described in this report, we identified a total of 38 cases of leukemia with MLL-AF10 fusion.
|
| 11106826 | Mutation | acute eosinophilic Leukemia; T Acute Lymphoblastic Leukemia | A translocation (10;11)(p12;q14) was observed in two children, one with acute eosinophilic leukemia and the other with acute T-cell lymphoblastic leukemia. The presence of CALM-AF10 fusion was ascertained by reverse transcriptase-polymerase chain reaction (RT-PCR) analysis. Fluorescence in situ hybridization (FISH) analysis showed that AF10 gene splitting was associated with partial inversion of chromosome 11 in the first patient
|
| 10997963 | Mutation | extramedullary Acute Myeloid Leukemia | A diagnosis of granulocytic sarcoma was made in a 2-year-old child based on the detection of myelomonocytic blasts in tissue obtained from a subcutaneous nodule with no evidence of concomitant disease in the bone marrow. The child responded to systemic chemotherapy and is in remission 3 years later. An identical clone with an in frame fusion of the MLL and AF10 genes was identified from both tissue and bone marrow samples.
|
| 10637483 | Mutation | Lymphoid Leukemia; Myeloid Leukemia | In order to understand the relationship between MLL, AF10, CALM and the leukemic process, fluorescence in situ hybridization and reverse transcriptase polymerase chain reaction were used to study a series of nine leukemia patients with a t(10;11). Six had myeloid leukemia (AML-M0, AML-M1, AML-M4 and AML-M5) and three had T cell lymphoblastic leukemia. We identified four different CALM/AF10 fusion products in five patients and AF10/CALM reciprocal message in one. We conclude that fusion of CALM and AF10 is a recurring abnormality in both lymphoid and myeloid leukemias of various types including AML-M5, and that the breakpoints in the two types of leukemia do not differ.
|
| 10637482 | Mutation | Acute Myeloid Leukemia | In this study we analyzed the CALM/AF10 and AF10/CALM fusion mRNAs in a series of three patients with AML, one patient with T-ALL and two patients with precusor T lymphoblastic lymphoma.
|
| 10221337 | Mutation | Leukemia | We performed reverse transcription-polymerase chain reaction and sequencing analysis on the t(10;11) leukemia samples obtained from four patients and one cell line, and we identified reciprocal fusion transcripts of AF10 and CALM in all the samples.
|
| 10554802 | Mutation | Leukemia | The CALM-AF10 fusion transcript was detected in all samples; however, the AF10-CALM fusion was not detected in two patient samples and one cell line. In RT-PCR analysis there were six isoforms of the CALM-AF10 fusion transcripts and five of AF10-CALM fusion transcripts. We also detected novel transcripts in U937. Sequence analysis revealed that all these isoforms had in-frame junctions and that some of them resulted from alternative splicing at different exons of CALM and others from different breakpoints at CALM and/or AF10. There were at least two different breakpoints of CALM and three of AF10 gene. Our results suggest that the CALM-AF10 fusion gene is a constant feature and is involved in the pathogenesis of haematological malignancies with t(10;11)(p13-14;q14-21), showing various and often multilineage phenotypes.
|